- Introduction to Subjective Methods
- Birth weight
- Body shape
- Weight and height
- Waist and hip circumference
- Introduction to Objective Methods
- Simple measures - stature
- Simple measures - weight
- Simple measures - circumference
- Simple measures - arm anthropometry
- Simple measures - skinfolds
- Simple measures - abdominal sagittal diameter
- Simple measures - head circumference
- Bioelectric impedance analysis
- Multi-component models
- Hydrostatic underwater weighing
- Air displacement plethysmography
- Hydrometry
- Whole body DEXA scan
- Near infrared interactance
- Whole body counting of total body potassium
- 3d photonic scan
- Magnetic resonance imaging (MRI) / Magnetic resonance spectroscopy (MRS)
- Total body electrical conductivity (TOBEC)
- Computed tomography (CT)
- Ultrasonography
- Introduction anthropometric indices
- Body mass index
- Fat and fat free mass indices
- Ponderal index
- Percentiles and Z-scores
- Anthropometry Video Resources
- Height procedure
- Protocol for measuring waist circumference
- Measuring hip circumference
- Weight and body composition procedure
Ultrasonography
Ultrasonography, or ultrasound, is an imaging technique and has been shown to be an alternative, non-invasive and reliable method to estimate visceral adipose tissue (VAT), subcutaneous adipose tissue (SCAT) and liver steatosis (fatty liver).
Ultrasound images are created by computer and digital memory from the transmission and reception of mechanical high-frequency waves applied through a transducer. The ultrasound waves spread through the body, producing an echo where density changes occur. For instance, in the case of human tissue, an echo is created where a signal passes from an adipose tissue region to a muscular tissue region. The echoes return to the transducer, where they are converted back into electrical signals that are amplified to form a digital image displayed on a monitor. Anthropometric outcomes assessed by USS (ultrasound scan) are summarised in Figure 1.
Visceral adipose tissue and abdominal subcutaneous adipose tissue protocol
The procedure for the VAT and SCAT assessment takes approximately 10 minutes and should be undertaken at the end of a quiet expiration.
The waist (the midpoint between the lower rib and the iliac crest) is measured using a D-loop tape measure with the participant standing. In infants, the waist is taken at the same location, but with the baby/toddler lying quietly in the supine position on a flat surface. On the skin, the location where the xiphoid line intercepts the waist circumference is marked. This is the area (midline position) where the transducer is positioned for the intra-abdominal (visceral) and abdominal subcutaneous fat measurements (see Figure 1 and 2).
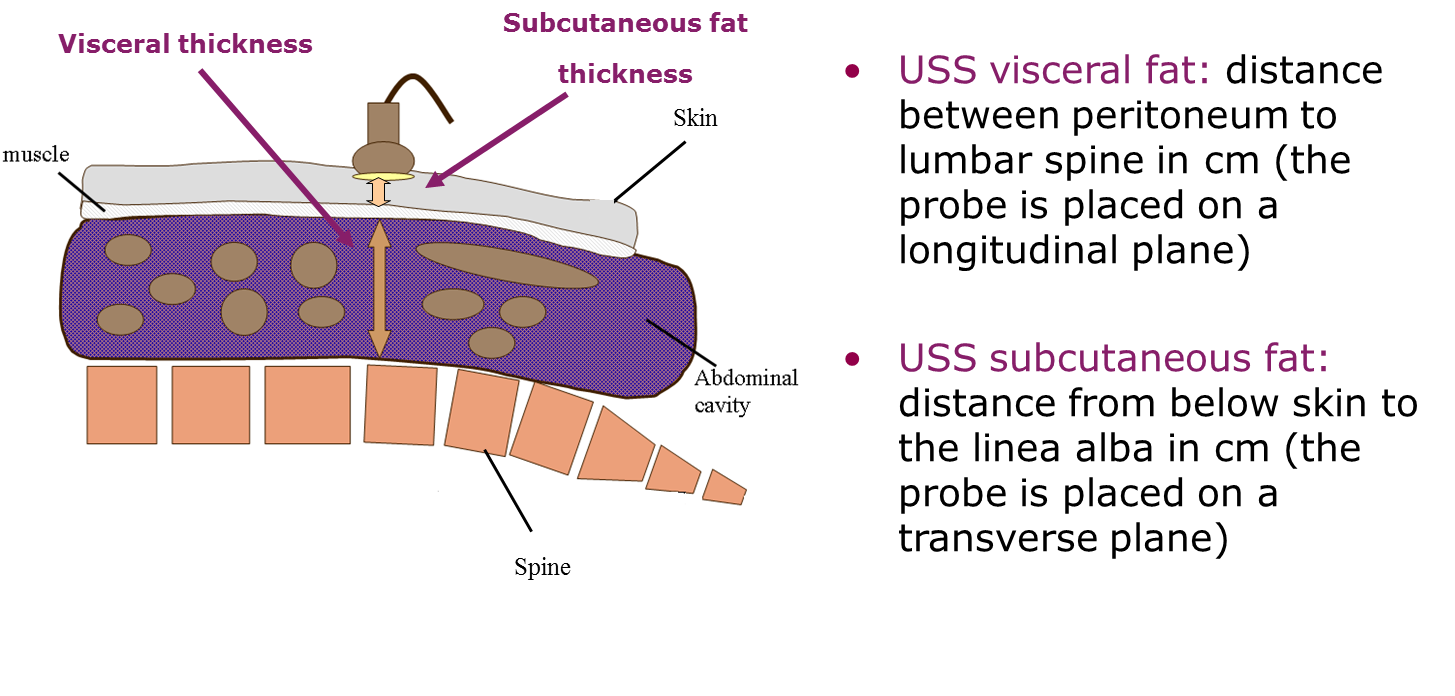
Figure 1 Ultrasound (USS) protocol for visceral fat and abdominal subcutaneous fat in both adults and young children.
Source: Professor Ronald Stolk, Groningen University, the Netherlands.
The visceral depth measurement is the distance taken from the peritoneum to the corpus of the lumbar vertebra.
- For this measurement, the depth of the ultrasound system is to be set at 7 cm in neonates and toddlers and 15 cm in adults and 20 cm in individuals with obesity (e.g. when the lumbar spine is not included in the scanning view).
- The transducer is positioned longitudinally on the midline location marked previously. Position the lumbar spine horizontally, and the measurement is captured without distortion of the abdominal cavity. To achieve this, decompress the transducer until there is no displacement of the abdominal cavity.
- On the captured image, the calipers are positioned perpendicularly in the centre of the image, and the distance between the peritoneum to the corpus of the lumbar vertebra is measured.
The subcutaneous depth measurement is the distance taken from the skin and the outer edge of the abdominal muscles (linea alba).
- For the subcutaneous measurement, the depth is to be set at 4 cm in neonates and toddlers and 9 cm in adults.
- The transducer is positioned transversely on the midline location marked previously. On the image, the linea alba is to be placed centrally. The transducer is then decompressed as much as possible to avoid compression of the subcutaneous tissue and the image is frozen just before the probe loses contact.
- On the captured image, position the calipers perpendicular in the centre of the image, and measure the distance between the skin and the outer edge of the abdominal muscles (linea alba).
There are other VAT protocols available in the literature:
- Where the VAT measurement is defined as the distance between the internal aspect of the rectus abdominis muscle and the posterior aortic wall in the abdominal midline. However, the aorta is not always clear in the images, especially in individuals with severe obesity. It may also be difficult to measure to the aorta, if it is located behind an air-filled intestine. Air-filled structures, such as the lungs or intestines, have low acoustic impedance, which means it reflects very little ‘echo’ or signal back to the transducer and the ultrasound images may be obscured due to these structures.
- Where specific VAT compartments are measured, such as pre-peritoneal (PP) fat pad. Although this measure has been validated against CT measures of PP rather than total VAT measure from CT. It is therefore only a proxy measure for VAT.
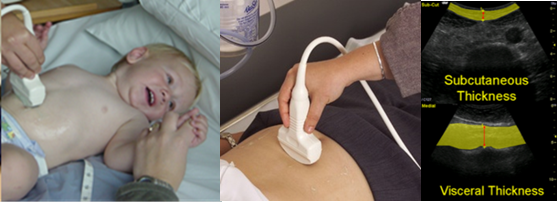
Figure 2 Ultrasound measurements and subcutaneous fat thickness and visceral fat thickness images.
Source: MRC Epidemiology Unit.
Subcutaneous adipose tissue assessment from other body locations
The procedure for the SCAT assessment depends on the number of body sites included in the protocol, but generally takes between 10-20 minutes. The transducer must be applied to the skin surface with uniform and constant pressure to prevent soft tissue compression. Like the skinfolds method, ultrasound measurements of SCAT thicknesses can be obtained from various body locations:
- Triceps
- Biceps
- Lateral forearm
- Anterior and posterior thigh
- Anterior and posterior calf
- Midthigh
- Calf
- Paraspinal (on back 2 cm superior to the iliac crest and 2 cm to the left of the midline)
- Suprailiac
- Sacral (the midline posteriorly just superior to the lumbar fossae)
- Subscapular
Liver steatosis protocol
- A sub-costal approach is used to image both liver lobes using a standard imaging protocol (see Figure 3).
- Depth is set at 15cm, gain is set at 40 and the focus is set at 5cm.
- The participant’s right arm is to be lifted above the head.
- The liver sweeps are made during deep inspiration.
- The first sweep is made from lateral to medial with the right lobe in an optimised longitudinal scan plane that shows the liver and the long axis of the kidney.
- The second sweep is made from cranial to caudal, starting at the level of the hepatic veins, along the portal vein towards the gallbladder, with the dome of the liver in a transverse position.
- The third sweep is a longitudinal scan of the left lobe and is made from lateral to medial just over the gallbladder area. The final sweep is a left transverse scan from the level of the hepatic veins towards the pancreas.
- Large individuals may need to be tilted to the side to be able to visualise the anatomy.
- If unable to view or obtain a clear image of the liver tissue, an intercostal approach may also be required.
- The procedure for the liver imaging is approximately 10-15 minutes.
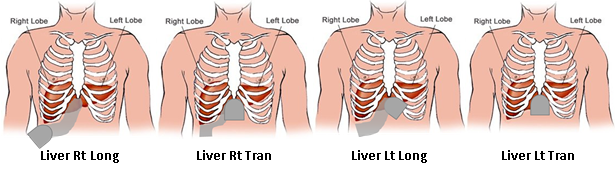
Figure 3 Liver protocol, showing the different liver sweeps and transducer position.
Source: Professor Ronald Stolk, Groningen University, the Netherlands.
The primary function of the ultrasound method is to assess soft tissue structures in clinical diagnoses. It is typically used in research settings to assess VAT, SCAT and liver steatosis when the reference methods, MRI and CT are not feasible.
It has been used in:
- Large-scale observational cohort studies
- Studies undertaking association analyses between exposure(s) and outcome(s)
- Interventions and randomised controlled trials to examine intervention or treatment efficacy (e.g. lifestyle intervention to modify regional fat depositions)
Visceral adipose tissue and abdominal subcutaneous adipose tissue output
Output is provided in the form of VAT and SCAT thicknesses from the images, which are either in mm or cm, depending on the ultrasound system’s settings. Prediction equations to estimate abdominal fat tissues are available in black South Africans and white older individuals. However their applicability may not be feasible in other populations.
Subcutaneous adipose tissue output from other body locations
Output is provided in the form of SCAT thicknesses from the images, which are either in mm or cm, depending on the ultrasound system’s settings. As with skinfolds, total body fat can be estimated using several measures of subcutaneous fat thickness at the various body locations using prediction equations.
Liver steatosis protocol
A semi-quantitative grading system is used to define normal echotexture (Figure 4) or mild, moderate and severe steatosis (Figure 5). The images can be scored at acquisition time if the operator is trained in the scoring protocol, or can be scored retrospectively.
The ultrasound images are scored according to standardised criteria, the liver steatosis scoring criteria are:
- Criterion 1 - increased echo reflectivity of the liver parenchyma (bright liver in comparison with the kidney)
- Criterion 2 - decreased visualisation of the intra-hepatic vasculature
- Criterion 3 - attenuation of ultrasound beam
Each criterion is scored on a 4-point scale (i.e. as 1, 2, 3 or 4) and summed, resulting in cumulative liver fat score which ranges from 3 to 12. A score of ≤4 is classified as normal liver, 5-7 as mild steatosis, 8-10 as moderate steatosis, and score ≥11 was classified as severe steatosis.
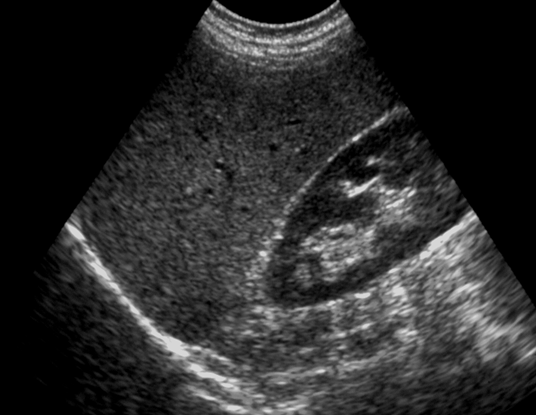
Figure 4 Normal liver echotexture. Longitudinal section through the right lobe of the liver. Similar echogenicity between liver parenchyma and the cortex of the right kidney. Intra-hepatic vascular anatomy is clearly visible and posterior aspects
of the liver are well depicted.
Source: MRC Epidemiology Unit.
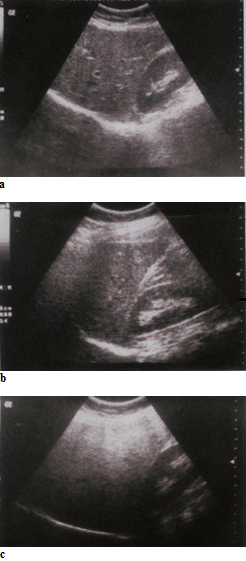
Figure 5 Gradation of hepatic steatosis: a) Mild - slightly increased echogenicity when compared to the renal cortex; blood vessels/diaphragm in view; b) Moderate - clear increased echogenicity of the liver parenchyma, impaired visualisation
of intrahepatic vascular anatomy, decreased echogenicity of the renal cortex; c) Severe - marked increased echogenicity/absorption, poor penetration and visualisation of intrahepatic vascular anatomy and diaphragm, marked decreased echogenicity of
the renal cortex.
Source: MRC Epidemiology Unit.
An overview of the characteristics of ultrasonography is summarised in Table 1.
Strengths
- It is a non-invasive, relatively inexpensive, widely available and reliable imaging technique for the quantification of abdominal fat depots (VAT and SCAT) and for the assessment of the presence of liver steatosis.
- It is applicable from infancy and in different ethnic groups when compared to direct imaging techniques.
- It is radiation free and it can be portable (Figure 6).
- It has been shown to be a better predictor of VAT and SCAT over and above simple anthropometry and DEXA.
- The association between visceral fat thickness measured by ultrasound and the metabolic risk factors of cardiovascular disease is more pronounced than the associations between these factors and waist or waist-to-hip ratio.
- Visceral fat thickness also to be associated with metabolic risk factors similar to that measured by abdominal CT scanning.
- It can be used from infancy, making this technique a feasible and valuable method for the assessment of possible risk factors associated with obesity in a very early stage.
- It is a valid method for detecting the presence or absence of liver steatosis when compared to the gold standard method (liver biopsy with histological or biochemical estimation of hepatic fat content) and the reference imaging methods, computer tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS). The sensitivity and specificity of ultrasound in diagnosing hepatic steatosis (presence vs not present) against MRS has been found to be 96% (95% CI: 87-99.6 %) and 94% (95% CI: 73-100%) respectively.
- It is a valid tool to assess subcutaneous fat thicknesses and is highly repeatable, with a precision similar to skinfolds.
- Ultrasonography has the advantage that measures can be taken in participant with severe obesity at sites not amenable to skinfold measures.
- Measures of total body fat using ultrasound SCAT thicknesses correlate well (r=0.88 to 0.95) with measures by hydrostatic weighing or MRI.
Limitations
- This method is subject to inter-observer variability [5, 6].
- Studies assessing the validity of ultrasonography to assess VAT and SCAT are unable to directly assess the “absolute validity” (the validity to assess absolute levels of VAT and SCAT from areas and volumes) between the criterion methods (MRI and CT) and ultrasonography as these measurements have different units. The USS thicknesses are one-dimensional (depth in cm) and the MRI and CT measures are two or three dimensional (areas in cm2 or volumes in cm3).
- Due to lack of evidence on absolute validity, ultrasonography should be used with caution when more accurate measures of fat distribution are required, for instance in individuals with specific conditions e.g. lipodystrophy. For these cases, the use of MRI and CT is still valuable.
- Requires trained operators.
- Ultrasound has low sensitivity in diagnosing mild steatosis and is prone to the operator’s subjective opinion.
Table 1 Characteristics of ultrasonagraphy.
| Characteristic | Comment |
|---|---|
| Number of participants | Large |
| Relative cost | Medium |
| Participant burden | Low |
| Researcher burden of data collection | Low |
| Researcher burden of coding and data analysis | Low to medium* |
| Risk of reactivity bias | Yes |
| Risk of recall bias | No |
| Risk of social desirability bias | No |
| Risk of observer bias | Medium to high** |
| Space required | Low*** |
| Availability | High |
| Suitability for field use | Medium |
| Participant literacy required | No |
| Cognitively demanding | No |
* The liver scoring might be tedious if images are reviewed retrospectively
**This depends on the systems
***Some systems are portable and they are as small as a laptop computer (Figure 6)
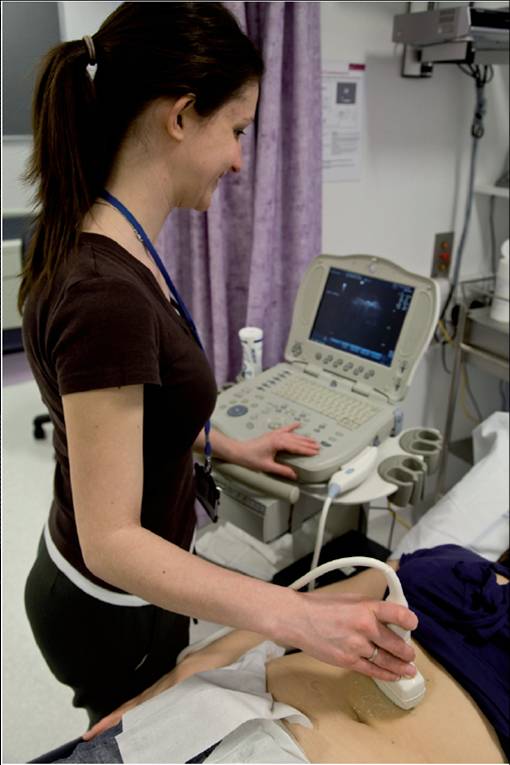
Figure 6 Portable ultrasound system.
Source: MRC Epidemiology Unit.
Considerations relating to the use of ultrasonography for anthropometry in specific populations are described in Table 2.
Table 2 Anthropometry by ultrasonography in different populations.
| Population | Comment |
|---|---|
| Pregnancy | Subcutaneous fat can be assessed throughout pregnancy; visceral fat thickness can be assessed during the first trimester (11-14 weeks’ gestation). |
| Infancy and lactation | Suitable. |
| Toddlers and young children | Distraction techniques may be required, e.g. having a projector with music can help settling the toddlers and are useful when taking these measurements in young children. |
| Adolescents | Suitable. |
| Adults | Suitable. |
| Older Adults | Suitable. |
| Ethnic groups | Suitable. |
| Other (obesity) | Suitable for most clinical populations but not for those that have a condition that affects levels of potassium. |
- It is recommended to estimate the relative inter and intra-observer technical error of measurement to monitor inter and intra-observer variability.
- Equipment performance to be assessed as part of a quality control program. This can also be arranged with the manufacturers.
Refer to section: practical considerations for objective anthropometry
- B mode Ultrasound system
- Trained operators
- Trained scorer (liver protocol only)
- Abdominal transducer (curved array transducer – with a frequency of 2-5 MHz this can be used for both VAT and abdominal SCAT; linear probes can be used to measure SCAT with a frequency of 5-7.5 MHz)
- Ultrasound Gel
- Ultrasound gel warmer
- D-loop tape measure
- Alcohol free germicidal disposable wipes
- Paper towels
- Ultrasound specialist from the manufacturer to optimise and adapt protocol settings
There are several commercial manufacturers of ultrasound systems. It is important to closely work with the ultrasound specialist from the company where the system has been purchased as the specialist will adapt the protocol settings to the specific instrument.
A method specific instrument library is being developed for this section. In the meantime, please refer to the overall instrument library page by clicking here to open in a new page.
Specialised software (e.g. ImageJ) has been developed in the past years for the quantitative assessment of liver steatosis using the hepatorenal index. This index is calculated on the basis of the ratio between the echogenicity of the liver and that of the right kidney cortex using histogram echo intensity. The software performs a histogram analysis of selected region of interests (ROI) of the kidney and liver and displays various statistics including mean brightness and size in pixels. Kaustubh et al has also demonstrated that this index can be directly calculated using DICOM (Digital Imaging and Communications in Medicine) from a PACS and a mark-up region of interest which is available from the NIH website.
CAP-coupled Fibroscan®, a technology based on liver fibrosis, has also been developed. The Controlled Attenuation Parameter (CAP) specifically targets liver steatosis using a process based on transient elastography. It measures the degree of ultrasound attenuation by hepatic fat at the central frequency of the FibroScan® M probe simultaneously with liver stiffness measurement (LSM).
- Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ. 1986;292(6512):13-5. Epub 1986/01/04.
- Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clinical radiology. 1991;43(1):26-31. Epub 1991/01/01.
- Mendler MH, Bouillet P, Le Sidaner A, Lavoine E, Labrousse F, Sautereau D, et al. Dual-energy CT in the diagnosis and quantification of fatty liver: limited clinical value in comparison to ultrasound scan and single-energy CT, with special reference to iron overload. Journal of hepatology. 1998;28(5):785-94. Epub 1998/06/13.
- Needleman L, Kurtz AB, Rifkin MD, Cooper HS, Pasto ME, Goldberg BB. Sonography of diffuse benign liver disease: accuracy of pattern recognition and grading. AJR American journal of roentgenology. 1986;146(5):1011-5. Epub 1986/05/01.
- Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082-90. Epub 2011/05/28.
- Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? European journal of gastroenterology & hepatology. 2003;15(5):539-43. Epub 2003/04/19.
- Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction Equations for Body Composition of Japanese Adults by B-mode Ultrasound. American Journal of Human Biology. 1994;6:161-70.
- Abe T, Tanaka F, Kawakami Y, Yoshikawa K, Fukunaga T. Total and segmental subcutaneous adipose tissue volume measured by ultrasound. Medicine and science in sports and exercise. 1996;28(7):908-12. Epub 1996/07/01.
- Armellini F, Zamboni M, Rigo L, Todesco T, Bergamo-Andreis IA, Procacci C, et al. The contribution of sonography to the measurement of intra-abdominal fat. Journal of clinical ultrasound : JCU. 1990;18(7):563-7. Epub 1990/09/01.
- Armellini F, Zamboni M, Robbi R, Todesco T, Rigo L, Bergamo-Andreis IA, et al. Total and intra-abdominal fat measurements by ultrasound and computerized tomography. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1993;17(4):209-14. Epub 1993/04/01.
- De Lucia Rolfe E, Modi N, Uthaya S, Hughes IA, Dunger DB, Acerini C, et al. Ultrasound estimates of visceral and subcutaneous-abdominal adipose tissues in infancy. Journal of obesity. 2013;2013:951954. Epub 2013/05/28.
- De Lucia Rolfe E, Norris SA, Sleigh A, Brage S, Dunger DB, Stolk RP, et al. Validation of ultrasound estimates of visceral fat in black South African adolescents. Obesity. 2011;19(9):1892-7. Epub 2011/07/09.
- De Lucia Rolfe E, Sleigh A, Finucane FM, Brage S, Stolk RP, Cooper C, et al. Ultrasound measurements of visceral and subcutaneous abdominal thickness to predict abdominal adiposity among older men and women. Obesity. 2010;18(3):625-31. Epub 2009/09/26.
- De Lucia Rolfe E, Brage S, Sleigh A, Finucane F, Griffin SJ, Wareham NJ, et al. Validity of ultrasonography to assess hepatic steatosis compared to magnetic resonance spectroscopy as a criterion method. Hepatology Communications: under review
- De Souza LR, Kogan E, Berger H, Alves JG, Lebovic G, Retnakaran R, et al. Abdominal adiposity and insulin resistance in early pregnancy. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2014;36(11):969-75. Epub 2015/01/13.
- Heymsfield SB, Lohman TG, Wang Z, Going SB. Human Body Composition. Second Edition, Human Kinetics, 2005.
- Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver international : official journal of the International Association for the Study of the Liver. 2012;32(6):902-10. Epub 2012/03/23
- Mook-Kanamori DO, Holzhauer S, Hollestein LM, Durmus B, Manniesing R, Koek M, et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound in medicine & biology. 2009;35(12):1938-46. Epub 2009/10/06.
- Shiralkar K, Johnson S, Bluth EI, Marshall RH, Dornelles A, Gulotta PM. Improved method for calculating hepatic steatosis using the hepatorenal index. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2015;34(6):1051-9. Epub 2015/05/28.
- Stolk RP, Meijer R, Mali WP, Grobbee DE, van der Graaf Y, Secondary Manifestations of Arterial Disease Study G. Ultrasound measurements of intraabdominal fat estimate the metabolic syndrome better than do measurements of waist circumference. The American journal of clinical nutrition. 2003;77(4):857-60. Epub 2003/03/29.
- Stolk RP, Wink O, Zelissen PM, Meijer R, van Gils AP, Grobbee DE. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(9):1346-51. Epub 2001/09/26.
- Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, et al. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR American journal of roentgenology. 2009;192(4):909-14. Epub 2009/03/24.
- Wu CH, Hu TH. Quantitative Assessment of Steatosis in Liver Tissue Using Controlled Attenuation Parameter Journal of Medical Ultrasound. 2014;22(3):131-2.
