- Nutrients, foods, and diets
- Dietary adequacy and nutritional requirements
- Diet variation, quantification, and misreporting
- Food composition data and databases
- Data processing
- Introduction to subjective measures
- Estimated food diaries
- Weighed food diaries
- 24-hour dietary recalls
- Food frequency questionnaires
- Diet checklists
- Diet histories
- Technology-assisted dietary assessment
Diet variation, quantification, and misreporting
What is diet variation and why is it important?
A key objective of many studies in terms of dietary assessment is to estimate the habitual dietary intake i.e. the average long-term intake of particular foods or nutrients for the group or individuals under study [1]. Habitual intake is exceedingly difficult to measure due to diet variation. The diet variation consists of:
- The differences in dietary intakes between different individuals (between-individual variation)
- The differences in dietary intakes for the same individual across time (within-individual variation)
Both the between- and within-individual diet variation should be considered during the selection of a dietary assessment method as well as in data analysis. Figure D.3.1 shows a hypothetical data for daily energy intake for five individuals across four days of dietary assessment. In the top figure (A), between-individual variation is large, as indicated by the spread of energy intakes across the five individuals (i.e. lines are relatively separated on the y-axis). On the other hand, within-individual variation is small, as indicated by stable energy intake across days (i.e. lines are relatively flat).
In the bottom figure (B), between-individual variation is small, as indicated by the lack of the spread of energy intakes across the five individuals (i.e. lines close together on the y-axis). In contrast, within-individual variation is large, as shown by the change in energy intake from day-to-day for each individual (i.e. lines are relatively uneven).
For the purpose of describing the prevalence of over- or under-nutrition in a population, understanding dietary variation is crucial. Bias in the estimation can occur without proper consideration of the within-individual diet variation. For example, if the within-individual variation is large and then between-individual variation is over-estimated, the estimate of the prevalence of over- or under-nutrition can be biased. Depending on the population average, the estimate of the prevalence can be either under-estimated or over-estimated (Figure D.3.1). The magnitude and direction of bias depends on the nutrient of interest, the method to assess the intake, and the target population.
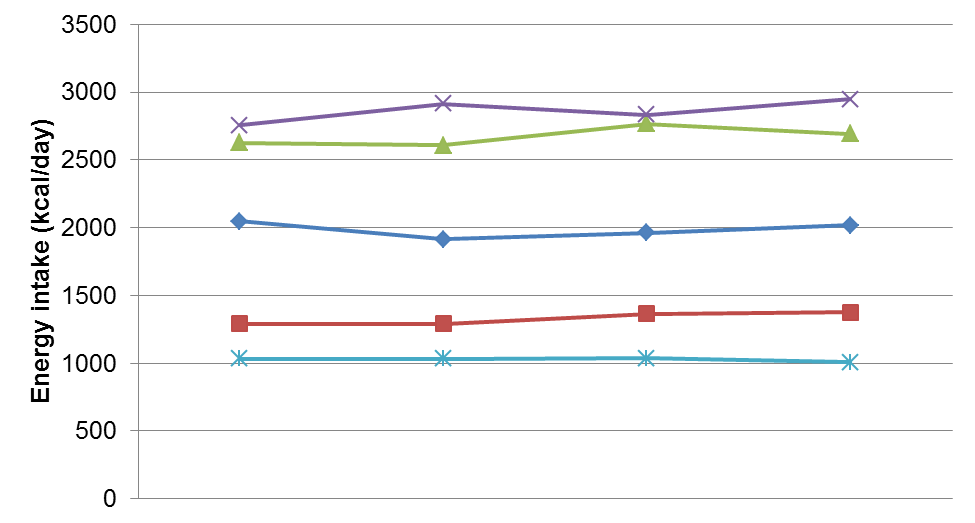
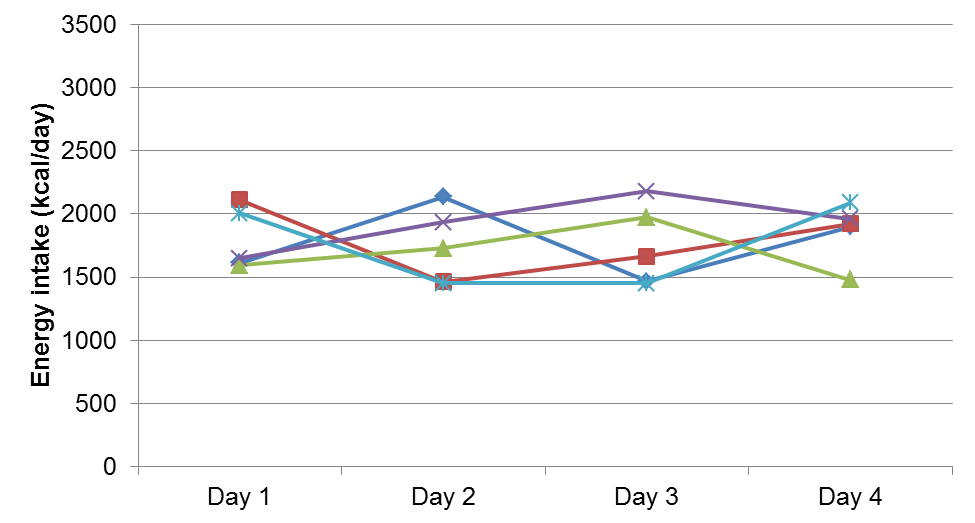
Figure D.3.1 Data demonstrating between- and within-individual variation in daily energy intake for five individuals across four days of measurement. Upper: Higher between-individual variation and lower within-individual variation. Lower: Higher within-individual and lower between-individual variation.
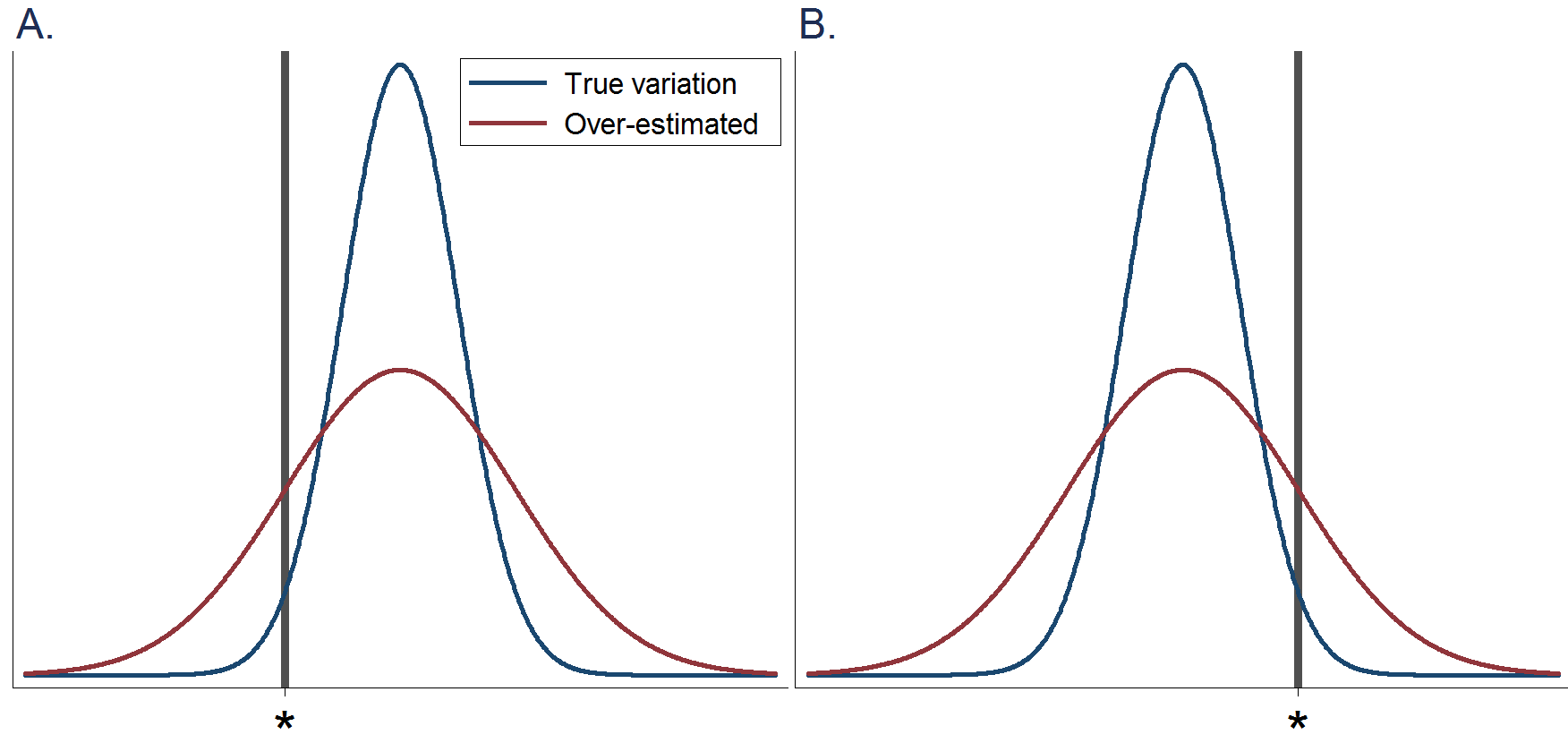
Figure D.3.2 Hypothetical histograms of nutrient intakes in a population. Blue lines indicate the true variation. Red lines indicate that the variation is over-estimated. The vertical line (*) indicates a cut-off point to define nutrient deficiency. A: When the population average is higher than a cut-off point for nutrient deficiency and when between-individual variation is over-estimated, the prevalence of individuals with nutrient deficiency is over-estimated. B: When the population average is lower than a cut-off point for nutrient deficiency and when between-individual variation is over-estimated, the prevalence of individuals with nutrient deficiency is under-estimated.
Example using the coefficient of variation
Dietary variation can be expressed as the coefficient of variation (CV), a standardised measure of spread that describes the amount of variability relative to the mean. The within-individual and between-individual variation can be expressed using CV as follows:
CV for within-individual variation = [(√within-individual variance) / mean]*100
CV for between-individual variation = [(√between-individual variance) / mean]*100
For most nutrients, within-individual variation is greater than between-individual variation [2]. This is illustrated in Table D.3.1 for a sub-group of Japanese women [1]. For example, fat intake was on average 59.7g per day, but this varied on average by 35% for each individual from one day to the next, but only by 19.3% from one individual to another. Figure D.3.1 shown above also displays the scenario that within-individual variation is greater than between-individual variation.
The relationship between within- and between-individual variation has important implications for assessment and can be summarised as a ratio (for example see variance ratio, ‘VR’, in Table D.3.1). If the within-individual variation is small relative to between-individual variation (Figure D.3.1), then individuals can be more readily distinguished [2,3]. If, as is normally the case, the within-individual variation is large relative to between-individual variation, then distinguishing between individuals and subsequent ranking is more difficult.
This partly explains why the mean intake of a group is usually more readily assessed than the intake in an individual.
Table D.3.1 Within- and between-individual variation in mean daily energy and nutrient intake of Japanese women (n=58).
| |
Mean | SD | CVw(%) | CVb(%) | VR |
| Energy (kcal) | 1824 | 327 | 20.6 | 17.2 | 1.44 |
| Protein (g) | 65.1 | 11.6 | 25.5 | 16.6 | 2.37 |
| Fat (g) | 59.7 | 12.6 | 35 | 19.3 | 3.28 |
| Carbohydrate (g) | 244 | 51 | 20.6 | 20.4 | 1.02 |
| Dietary fibre (g) | 12.4 | 3.2 | 33.8 | 24.8 | 1.86 |
CVw = within-individual coefficient variation
CVb = between-individual coefficient variation
VR = within-individual to between-individual variance ratio. A larger VR indicates within-individual variation is large compared to between-individual variation.
Adapted
from: [1].
Factors influencing diet variation
Diet variation can be influenced by the following factors:
- Seasonal variation. Dietary assessment undertaken in a few weeks of a year may give a distorted view of habitual micronutrient intake, for example, vitamin C mainly from seasonal fruits in a population. On the other hand, seasonal variation is of less relevance than in the past in some countries and settings where large amounts of imported fruits and vegetables are available. The costs of foods may also vary with their seasonal availability. This issue should be considered especially relative to the population under study.
- Variation in meal patterns.
- Variation in household composition.
- The wide range of foods available.
- Consumption of food outside the home (e.g. takeaways, restaurants).
- Working patterns.
- Special days, including festivities etc.
- Disease states.
Accounting for diet variation
Study sample
Both the within-individual and between-individual diet variation are natural phenomena. Therefore, regardless of the sample size of a study, we cannot reduce the diet variation. However, if we increase the sample sizes of individuals and repeats of dietary assessment, we can improve estimates (i.e. increase precision) of the within-individual variation, the between-individual variation, or both.
To estimate the within-individual variation and improve the estimates, we need to increase the sample size of repeats from the same participants. To do the same for the between-individual variation, we need to increase the sample size of study participants (i.e. include a greater number of participants). Of note, if we have a huge sample size of study participants, but dietary intakes are assessed only once, we cannot estimate the within-individual variation of the participants.
Selection of dietary assessment method
- Dietary history: assesses the between-individual variation and the usual intake, while it depends on the reference period of the history assessed.
- Food frequency questionnaire (FFQ): assesses the between-individual variation and the usual intake, while it depends on the reference period of the questionnaire.
- Weighed or estimated food diaries: assess the between-individual variation and if done on multiple days, they also assess the within-individual variation and it is more probable that they capture the habitual diet.
- 24-hour recalls: assess the between-individual variation and if done on multiple days, they also assess the within-individual variation and it is more probable that they capture the habitual diet.
- Biomarkers of intake: assess the between-individual variation and if there are repeated measures for them, they also assess the within-individual variation.
Time of the year
The greater the seasonal variation, the broader the reference period should be when referring to FFQ; or repeated FFQs should be undertaken to cover the usual diet over seasons. When referring to diet records or 24-hour recalls, the greater the seasonal variation, the more distributed the days across the year should be.
If a study plans to assess dietary change over years, investigators may want to collect repeated dietary data at the same time of the year to consistently account for seasonal variation. If the repeated measures were collected in different seasons, changes in dietary intakes would be confounded by the within-individual seasonal variation.
Number of days for multiple dietary records and 24-hour recalls
The factors which should be considered to decide the number of days include the following:
- Within-individual variation [4]: The greater the day-to-day variation in intake, the more days are needed to accurately capture habitual intake. Macronutrients require fewer recording days than micronutrients in general. The number of days required to capture habitual micronutrient intakes depends on the amounts of the nutrients contained in food e.g. omega 3 fatty acids or vitamin A, and the frequency with which these foods are consumed. The lower the amounts and the frequency, the longer the period of assessment required. The use of a complementary dietary assessment method such as an FFQ may help to better capture infrequently consumed food groups [5]. An increased number of days can affect both recruitment and compliance so a trade-off is required between the number of days and participant burden. In the UK, for instance, the current National Diet and Nutrition Survey (NDNS) has been reduced from seven days of assessment to four days (and changed from weighed to estimated records) to improve its recruitment and completion.
- The main interest of the study: if the interest is at an individual level, more days are needed to account for within-individual variation. If the interest is at a group level, fewer days might be needed. If mean intakes of individuals are required, multiple days of recording are required, e.g. for 90% of the population, 13 days of recording are necessary to determine the mean energy intake with a standard error of ± 10% [5]. Further estimates of the number of days required to rank dietary intake are provided by Erkkola et al. [4].
Day of the week
The biggest difference in dietary intake has often been assumed to be between weekdays and weekends. The assumption often made is that diets on Saturday and Sunday were similar. However, analysis from the UK National Diet and Nutrition Surveys (NDNS) has shown that these two days may vary greatly, suggesting that both days may need to be assessed [6,7].
In studies using interviewer-administered dietary assessment, attention must be paid to the days of the week. Interviewers who do not work weekends or respondents who do not want to be interviewed on weekends for instance may lead to a bias in the dietary data collected.
In the estimation of the habitual consumption of dietary intakes from multiple days of dietary records or 24-hour recalls, a simple average can cause bias. Suppose dietary assessment is implemented on one day from the weekend and two days from weekdays. The average of three days over-represents the weekend because 33% of data come from weekends, rather than 28.6% (2 days out of 7 days). Unless a 7-day assessment is implemented, a weighted average should be calculated. In this example, the calculation should be (2 x weekend + 5/2 x (weekday 1 + weekday 2)) divided by 7.
In the analysis of 24-hour recall data, days of recording and days of diets should be carefully distinguished. For instance, dietary data collected on Monday represents recalled diet on a Sunday.
Why is portion size important?
The estimation of a portion size has been recognised for more than 50 years as a source of error in studies measuring dietary intake [8]. A coefficient of variation of the differences between estimates and weights of food portions has been consistently shown to be around 50% for foods and 20% for nutrients [9]. The errors associated with quantifying the portion of food consumed represent probably the largest measurement error in most dietary assessment methods [2,3].
Estimating portion size
Options for estimating portion size include:
Weighing
Portion sizes and leftovers are measured by a participant or research staff. Weighing increases the burden of data collection and is still subject to error.
Household measures
Household measures (e.g. spoon sizes) may be associated with errors and considerable variations. The discrepancies in individual ability to estimate food portions with the use of household measures have been shown to be independent of age, body weight, social status and sex but shown to vary with the food type and true portion size [10].
Food photographs (2 dimensional)
Food photographs [11,12] are the most commonly used tool to assist the estimation of portion sizes. Showing photos in a computer display is likely to be helpful in an interview-assisted or a computer-based self-administered dietary assessment. However, studies have shown that both adults and children would find it challenging to estimate portion sizes using photographs, and improved portion size assessment aids are required for all age groups [13]. Some foods have been reported to cause greater difficulty than others; for instance, most of the food categories were underestimated (ranging from −2.3% for cassava to −6.8% for rice), except for beverages (+1.6%) and leafy vegetables (+8.7%) which were somewhat overestimated [14].
Photographs that depict a portion (amount consumed), or serving size (amount served), are typically presented as a series of graduated photographs (see Figure D.3.3) for each food item, bound together in an atlas [15].
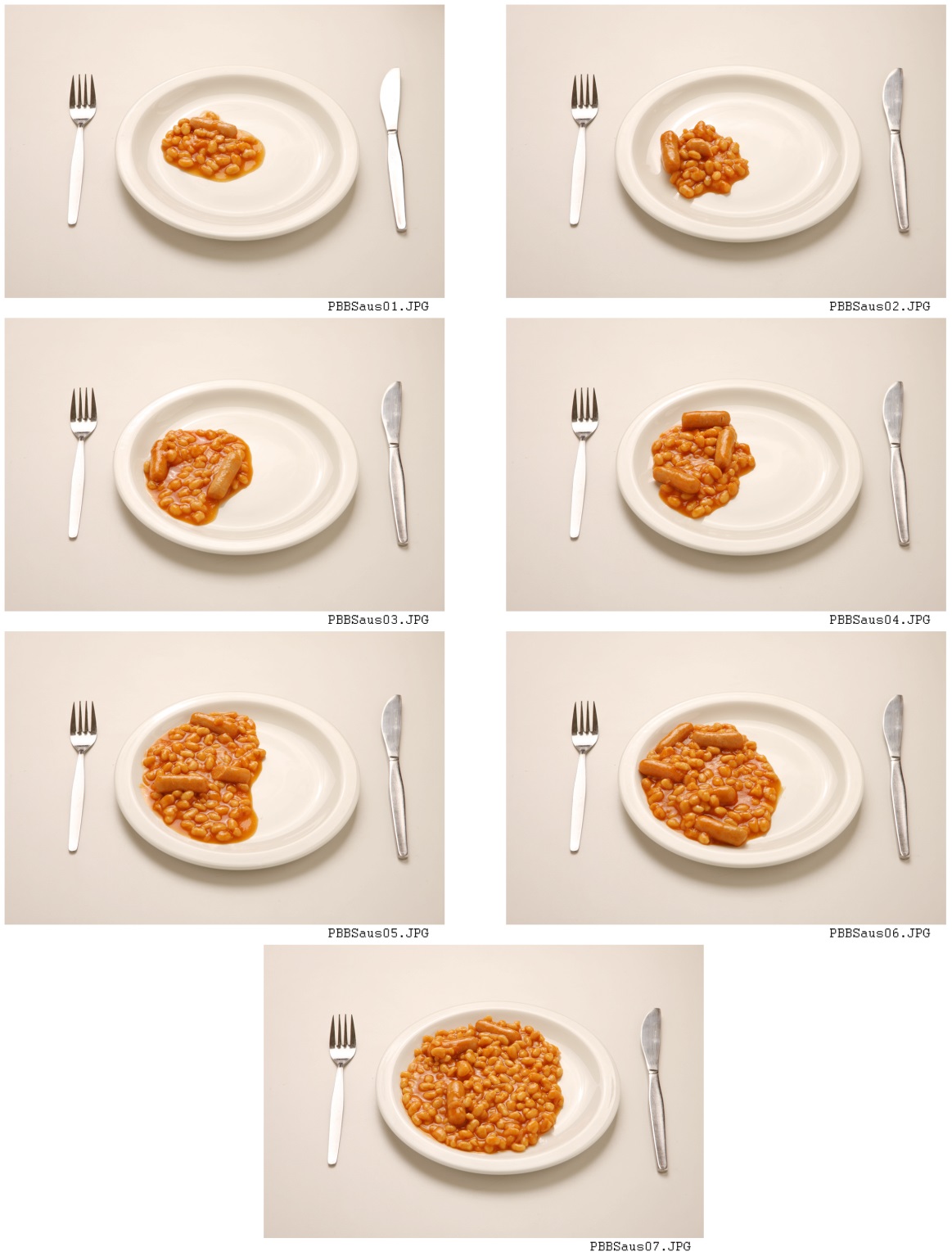
Figure D.3.3 Example of graduated food photographs used to assess portion size with children aged 18 months to 16 years.
Source: [6].
Developing a food atlas is a time-consuming process [17], so where possible an existing tool should be used, providing it has been validated in a similar population. Some factors which should be carefully considered are [17]:
- The size of the image.
- The number and the range of portion sizes. Multiple photographs seem to result in more accurate estimates.
- The angle at which the photograph is taken.
- Whether a colour or black and white photograph is used.
- Whether other objects are used to give a sense of scale; this may be helpful.
- The way the food is presented.
- The population of interest. It is important that the portion sizes in photographs are based on contemporary data in a similar population. One study has shown that children overestimated food weight by 46% on average, using adult food photographs but this was reduced to an over-estimation of 7% when age-appropriate photographs were provided. Adults overestimated portions by 18% using the adult food photographs [18].
A useful description is available [19] of how food photograph portions were developed using data from previous National Diet and Nutrition Surveys (NDNS) in the UK in children and young people. In this study, food photographs were compared to a computer tool, the interactive portion size assessment (IPSAS), and found to be comparable with good accuracy but poor precision; making it suitable for group estimates not individual estimates.
Food replicas (3 dimensional) and food models (3 dimensional)
Canada is one of the first countries that used the collection of three-dimensional food models in its national nutrition survey [15]. Other countries have also included such an aiding tool, e.g. the United States and New Zealand. The United States National Health and Nutrition Examination Survey (NHANES) uses 3-dimensional measurement aids in the dietary component of the survey. A few studies that compared the use of 2- and 3-dimensional food models suggested that 2-dimensional ones are as effective as 3-dimensional ones [20,21]. Other studies evaluating food portion aids have shown mixed results and many studies have been carried out in highly controlled and non-representative conditions [14]. Food models are likely to be more reliable than household measures, but often only one size is available, introducing bias as individuals report specific portions that agree with the models available.
Average portion sizes for a given population
Average portion sizes for some foods can be estimated by using existing data [22,23]. These data should ideally be kept up to date and be specific to a study population. Standard portion sizes are used in ascribing portions in FFQs. Respondents completing semi-quantitative FFQs may often have difficulty in relating their consumption to pre-defined reference portion sizes [24].
Factors that influence the estimation of portion size
The following factors may influence estimates of portion size [25]:
Food characteristics
- Foods of indeterminate shape tend to be associated with larger errors and liquids with smaller errors, otherwise, there are no consistent observations.
- Larger portion sizes appear to be more difficult to measure than small portion sizes.
Individual characteristics
The following individual characteristics can influence the estimation of portion size [12,1]:
- Perception - the individual’s ability to relate an amount of food present in reality to the amount depicted in a photograph.
- Conceptualisation - the ability of an individual to make a mental construction of an amount not present in reality and to relate that to a photograph.
- Memory - which affects the precision of the conceptualisation process.
- Women have, on occasion, been shown to provide better estimates than men; this is likely due to their greater role in food preparation.
- Training of the respondents might reduce the incurred error.
What is misreporting?
Misreporting is a form of respondent bias documented in studies using subjective methods of dietary assessment, and includes both under- and over-reporting.
The detection of misreporting is an investigation of the validity of the dietary data obtained. Often energy intake is used as a proxy for dietary intake in investigations because if energy intake is underestimated, intakes of the other nutrients may also be underestimated [25]. Assessment of the validity of dietary assessment methods therefore often concentrates on estimates of energy expenditure. The development of doubly labelled water (DLW) as a gold standard measure of energy expenditure was thus fundamental to advancing this area of dietary assessment and its links with health outcomes such as obesity [26].
Misreporting in dietary assessment was extensively studied during the late 1980s/early 1990s. At that time, the focus of obesity research was on identifying a metabolic cause of obesity, as self-reported dietary intakes did not show intakes that were higher than in lean counterparts [27].
In recent decades, diet quality, in addition to quantity, has received much attention. To study diet quality independent of diet quantity in population-health research, overall dietary habits and dietary intakes have been assessed after adjustment for total energy intake. Therefore, although the literature on misreporting largely focuses on the validity of total energy intake, it is crucial to consider the validity of estimating other dietary intakes after adjusting for total energy intake.
What is the profile of misreporters?
Characteristics of misreporters are population-specific and have not been consistent in the published literature [28,29]. Individuals who misreport on one occasion are likely to do so on subsequent occasions, therefore repeated or more prolonged periods of assessment may not reduce the error due to misreporting [30]. Characteristics that influence degrees of misreporting have been studied and include some features as summarised below [31-39]:
- Perception of social desirability plays an important role in misreporting.
- Body size measured by the body mass index (BMI) seems to be the most consistent determinant of energy misreporting in the use of subjective methods of dietary assessment such as FFQ and 24-hour recall.
- Participants may tend to misreport dietary intakes. By participating in a study as a volunteer after being informed of a study aim, study participants may make a behavioural change during the period of dietary assessment (reactivity bias). Misreporting due to reactivity bias may be more substantial among adults with obesity or overweight who underreport more than lean counterparts.
- Other potential determinants of misreporting that have been reported in the literature but show lower consistency include age, sex/gender, education level, smoking, dietary behaviours such as restrictive eating, emotional eating, eating outside of home and knowledge on healthy eating, BMI-related factors such as body image, dieting, weight changes over years, physical activity, body fat, and disease status.
Implications of misreporting
Misreporting can potentially lead to the following:
- Bias in any diet and disease relationships, particularly in the study of obesity [25].
- Limited and insufficient definition of food patterns and the subsequent derivation of food-based guidelines [40].
- Estimates of different food intakes are affected differentially, with healthy foods often overreported and unhealthy foods often underreported [32].
Accounting for misreporting
Doing nothing may be considered an option, with caveats
If the research aim is to rank individuals and the ability of ranking individuals is well supported objectively (e.g. in a validation study), systematic misreporting can be ignored in data analysis. For example, if 100% of study participants underestimate fruits and vegetable consumption by 10%, the rank of the participants by the consumption is stable. Similarly, the association of dietary intakes with a health outcome can be unchanged even in presence of over- or under-reporters (refer to systematic errors page). Therefore, the need for and approach to accounting for misreporting should be considered with a research aim.
It is practically difficult to ensure that systematic errors are not differential. Although doing nothing is an option, analysis after accounting for possible over- and under-reporting of total energy intake is generally recommended in dietary research.
Estimation of energy intake with reference to estimated total energy expenditure
As noted above, a focus on misreporting tends to be on the estimation of absolute levels of total energy intake. Habitual total energy intake should match with habitual total energy expenditure under the assumption that participant weights are static (in a state of energy balance). Under this assumption, estimates of total energy expenditure are often used to identify mis-reporters in dietary research.
Ideally, studies would measure total energy expenditure (TEE) by doubly labelled water (DLW), but this is expensive (approx. £1000 per dose). Therefore, a typical population-health study estimates TEE by basic characteristics that are readily measurable. The estimation uses standard equations of basal metabolic rate (BMR) [41], plus energy expenditure due to physical activity level (PAL). Estimates of TEE are improved if physical activity is assessed objectively. BMR can be measured directly with calorimetry.
TEE comprises energy expenditure due to physical activity and also digestion of meals. The 'thermic effect of food’ can be estimated from dietary consumption, but is typically ignored because TEE, used as a reference to subjective total energy intake, should be independent of dietary measures assessed simultaneously. Instead, PAL is assigned to be a constant coefficient that captures energy expenditure in addition to BMR. Calculated from DLW-derived TEE, PALs are assumed to be as detailed in Table D.3.2.
Table D.3.2 Physical activity levels derived from doubly labelled water measurement.
| Activity | Physical activity level (PAL) |
|---|---|
| Chair-bound or bed-bound |
|
| Seated work with no option of moving around and little or no strenuous leisure activity |
|
| Seated work with discretion and requirement to move around but with little or no strenuous leisure activity |
|
| Standing work |
|
| Significant amounts of sport or strenuous leisure activity (30-60 min, 4-5 times per week) |
|
| Strenuous work or very active leisure |
|
In the literature, the use of TEE estimates is inconsistent and a topic of methodological research [42-46]. The following are examples of methods to distinguish individuals who are under-reporters, plausible reporters and over-reporters or to control for the magnitude of over- and under-reporting.
I. The ratio of total energy intake to TEE
Different approaches of using the ratio can be undertaken. A study may exclude individuals based on certain cut-points defined within that study, for example, bottom and/or top percentiles and means ±1 standard deviation (Note the standard deviation represents within-individual variation or uncertainty of the ratio). Some more recent studies have suggested statistical adjustment for the ratio to hold degrees of misreporting consistent in a population and stratification of results based on misreporting status to avoid unnecessary loss of power and introduction of unpredictable selection bias [43,45,46].
Using the ratio of total energy intake to TEE, when examining relationships between diet and health in a nationwide cohort in the United States, the use of ±1 SD cut-offs was reported to be preferable to the ±2 SD cut-offs for excluding inaccurate reports [42]. Mendez et al. [43] reported that the use of such alternative methods had a stronger influence on associations compared to using the Goldberg method for instance in the Spanish EPIC cohort.
II. Goldberg cut-offs
Goldberg cut-offs have been used in dietary surveys or studies that aim to assess absolute levels of total energy intake and other dietary components. Goldberg and colleagues [47] developed two cut-offs for the agreement between PAL and the ratio of reported energy intake to BMR. The application was first demonstrated by Black et al. [3]. In the first, ‘CUT-OFF 1’, PAL was set at 1.35, the minimum plausible value for most individuals who are weight stable. Subsequent work in this area led to a recommendation that this cut-off should no longer be used because it fails to account for biological variability and measurement error in estimating both energy intake and expenditure [48]. It was also noted that it underestimated underreporting in individuals whose activity was above a sedentary level.
‘CUT-OFF 2’ differs from the previous cut-off as total energy expenditure or PAL varies according to the population or the individuals under study. As with CUT-OFF 1 it involves a statistical comparison between reported energy intake and BMR accounting for biological variability and measurement error. Originally, only a lower cut-off was developed, but if the activity is known or can be assumed, an upper limit can be determined [48], i.e. the 95% confidence limits for the ratio of reported energy intake to BMR and PAL.
Researchers should state the criteria used for calculating the cut-offs, and include such information in any publications, as there is potential for inappropriate use of the cut-offs, through a lack of thorough understanding of the principles [25,42].
III. Adjustment for predictors of energy balance such as BMI
More recent studies have suggested potential bias when the ratio of estimated energy intake to TEE was used in analyses and when a study outcome is correlated with TEE such as anthropometric outcomes (e.g. fat mass, fat-free mass). An alternative proposed is the adjustment for predictors of energy balance. This approach has been shown to result in associations with energy intake closer to those derived from an objective measure of energy intake [39].
For misreporting of dietary intakes, rather than total energy intake, bias due to misreporting could be reduced in a population through adjustment for total energy intake [49]. This relates to the topic of errors in multiple variables (e.g. food consumption and energy intake).
IV. Use of predefined lower and upper limits of energy intake
To estimate TEE without an objective measurement (e.g. DLW), equations involve the use of information on weight and height. If the weight is not objectively measured, weight may be over- or under-estimated, and TEE estimation can be biased and not useful to identify misreporters. To avoid this issue, a recent study proposed predefined population-specific limits of energy intake to identify misreporters, such as energy intake <500 and >3,500 kcal/day for women [44].
Recommendations
- Conduct or refer to a validation study in which systematic and random errors should be observed to be minimal after accounting for possible misreporters.
- Cut-offs can provide help for identifying under-reporters.
- Measure physical activity, ideally by an objective method such as accelerometry, to improve estimates of energy expenditure. Alternatively, subjective methods such as a physical activity questionnaire can be used to rank individuals according to activity level but have limited validity for estimating the absolute energy expenditure of individuals.
- Assess dietary restraint if possible.
- Any researcher working with study individuals must be aware that their attitude, general demeanour, and non-verbal and verbal language convey messages which may affect the likelihood of misreporting, such as through social desirability bias.
- Conducting focus groups to explore behaviours around dietary assessment may provide an insight into the likelihood of misreporting [8].
- Conduct additional analyses using different approaches to accounting for misreporting (i.e. sensitivity analysis) to assess the stability of results.
- Dekkers AL, Verkaik-Kloosterman J, van Rossum CT, Ocké MC, SPADE, a new statistical program to estimate habitual dietary intake from multiple food sources and dietary supplements. J Nutr, 2014;144:2083-91
- Fukumoto A, Asakura K, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, Todoriki H, Miura A, Fukui M, Date C. Within- and between-individual variation in energy and nutrient intake in Japanese adults: effect of age and sex differences on group size and number of records required for adequate dietary assessment. J Epidemiol. 2013;23(3):178-86. doi: 10.2188/jea.je20120106. PMID: 23583922; PMCID: PMC3700253.
- Black AE, Goldberg GR, Jebb SA, Livingstone MB, Cole TJ, Prentice AM, Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur J Clin Nutr, 1991;45:583-99
- Erkkola M, Kyttälä P, Takkinen H, Kronberg-Kippilä C, Nevalainen J, Simell O, Ilonen J, Veijola R, Knip M, Virtanen S. Nutrient intake variability and number of days needed to assess intake in preschool children. Br J Nutr, 2011;106(1):130-140. doi:10.1017/S0007114510005167
- Dao MC, Subar AF, Warthon-Medina M, Cade JE, Burrows T, Golley RK, Forouhi NG, Pearce M, Holmes BA. Dietary assessment toolkits: an overview. Public Health Nutr. 2019;22(3):404-418. doi: 10.1017/S1368980018002951. PMID: 30428939; PMCID: PMC6368251.
- Whitton C, Nicholson SK, Roberts C, Prynne CJ, Pot GK, Olson A, Fitt E, Cole D, Teucher B, Bates B, Henderson H, Pigott S, Deverill C, Swan G, Stephen AM. National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br J Nutr. 2011;106(12):1899-914. doi: 10.1017/S0007114511002340. PMID: 21736781; PMCID: PMC3328127.
- Champagne CM, Han H, Bajpeyi S, Rood J, Johnson WD, Lammi-Keefe CJ, Flatt JP, Bray GA. Day-to-day variation in food intake and energy expenditure in healthy women: the Dietitian II Study. J Acad Nutr Diet. 2013;113(11):1532-1538. doi: 10.1016/j.jand.2013.07.001. PMID: 24021734.
- Young CM, Chalmers FW, Church HN, Clayton MM, Murphy GC, Tucker RE. Subjects' estimation of food intake and calculated nutritive value of the diet. J Am Diet Assoc. 1953;29(12):1216-20.
- Bingham SA, Nelson M. Assessment of food consumption and nutrient intake In: Margetts BM, Nelson M, editors. Design Concepts in Nutritional Epidemiology Oxford: Oxford University Press; 1991.
- Bucher, T., Weltert, M., Rollo, M. et al. The international food unit: a new measurement aid that can improve portion size estimation. Int J Behav Nutr Phys Act 14, 124 (2017). https://doi.org/10.1186/s12966-017-0583-y
- Ferreira GR, da Silva DG, Taconeli CA, Pierin GAF, Lass HdS, Martins LS, et al. Assessment of bias and associated factors for food portion quantification with photos in Brazil. Measurement: Food. 2021;3:100007.
- Naska, A., Valanou, E., Peppa, E., Katsoulis, M., Barbouni, A., Trichopoulou, A. Evaluation of a digital food photography atlas used as portion size measurement aid in dietary surveys in Greece. Public Health Nutr, 2016;19(13):2369-2376. doi:10.1017/S1368980016000227
- Timon, C.M., Cooper, S.E., Barker, M.E. et al. A comparison of food portion size estimation by older adults, young adults and nutritionists. J Nutr Health Aging 2018;22:230–236. doi:10.1007/s12603-017-0937-9
- Lazarte, C.E., Encinas, M.E., Alegre, C. et al. Validation of digital photographs, as a tool in 24-h recall, for the improvement of dietary assessment among rural populations in developing countries. Nutr J 2012;11:61. doi:10.1186/1475-2891-11-61
- Hack S, Jessri M, L'Abbé MR. Evaluating Diet Quality of Canadian Adults Using Health Canada's Surveillance Tool Tier System: Findings from the 2015 Canadian Community Health Survey-Nutrition. Nutrients. 2020;12(4):1113. doi: 10.3390/nu12041113. PMID: 32316338; PMCID: PMC7230800.
- Foster E, Hawkins A, Barton KL, Stamp E, Matthews JNS, Adamson AJ. Development of food photographs for use with children aged 18 months to 16 years: Comparison against weighed food diaries – The Young Person’s Food Atlas (UK). PLOS ONE. 2017;12(2):e0169084.
- Nikolić M, Milešević J, Zeković M, Gurinović M, Glibetić M. The Development and Validation of Food Atlas for Portion Size Estimation in the Balkan Region. Front Nutr. 2018;5:78. doi: 10.3389/fnut.2018.00078. PMID: 30271776; PMCID: PMC6146086.
- Foster E, Matthews JN, Nelson M, Harris JM, Mathers JC, Adamson AJ. Accuracy of estimates of food portion size using food photographs--the importance of using age-appropriate tools. Public Health Nutr. 2006;9(4):509-14.
- Foster E, Matthews JN, Lloyd J, Marshall L, Mathers JC, Nelson M, et al. Children's estimates of food portion size: the development and evaluation of three portion size assessment tools for use with children. Br J Nutr. 2008;99(1):175-84.
- Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655-66.
- Posner BM, Smigelski C, Duggal A, Morgan JL, Cobb J, Cupples LA. Validation of two-dimensional models for estimation of portion size in nutrition research. J Am Diet Assoc. 1992;92(6):738-41.
- Hotz C, Abdelrahman L. Simple methods to obtain food listing and portion size distribution estimates for use in semi-quantitative dietary assessment methods. PLoS One. 2019;14(10):e0217379. doi: 10.1371/journal.pone.0217379. PMID: 31626670; PMCID: PMC6799923.
- Wrieden WL, Longbottom PJ, Adamson AJ, Ogston SA, Payne A, Haleem MA, et al. Estimation of typical food portion sizes for children of different ages in Great Britain. Br J Nutr. 2008;99(6):1344-53.
- Benton D. Portion size: what we know and what we need to know. Crit Rev Food Sci Nutr. 2015;55(7):988-1004. doi: 10.1080/10408398.2012.679980. PMID: 24915353; PMCID: PMC4337741.
- Banna JC, McCrory MA, Fialkowski MK, Boushey C, Examining Plausibility of Self-Reported Energy Intake Data: Considerations for Method Selection. Frontiers in Nutrition, 2017;4:45. doi: 10.3389/fnut.2017.00045
- Westerterp KR. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol. 2017;117(7):1277-1285. doi: 10.1007/s00421-017-3641-x PMID: 28508113; PMCID: PMC5486561.
- Prentice AM, Black AE, Coward WA, Davies HL, Goldberg GR, Murgatroyd PR, et al. High levels of energy expenditure in obese women. Br Med J. 1986;292(6526):983-7.
- Lo Siou, G., Akawung, A.K., Solbak, N.M. et al. The effect of different methods to identify, and scenarios used to address energy intake misestimation on dietary patterns derived by cluster analysis. Nutr J, 2021;20:42 doi:10.1186/s12937-021-00696-3
- Tooze JA, Freedman LS, Carroll RJ, Midthune D, Kipnis V. The impact of stratification by implausible energy reporting status on estimates of diet-health relationships. Biom J. 2016;58(6):1538-51.
- Curtis K, Atkins L, Brown K, Big hearts, small hands: a focus group study exploring parental food portion behaviours. BMC Public Health. 2016;17:716
- Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL, Ehlers M, Midthune D, Williams GC, Greene GW, et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr, 2007;138:226S-234S
- Jessri M, Lou WY, L'Abbé MR, Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey. Br J Nutr, 2015;115:147-59
- Mossavar-Rahmani Y, Tinker LF, Huang Y, Neuhouser ML, McCann SE, Seguin RA, Vitolins MZ, Curb JD, Prentice RL, Factors relating to eating style, social desirability, body image and eating meals at home increase the precision of calibration equations correcting self-report measures of diet using recovery biomarkers: findings from the Women's Health Initiative. Nutr J. 2012;12:63
- Murakami K, Livingstone MB, Prevalence and characteristics of misreporting of energy intake in US adults: NHANES 2003-2012. Br J Nutr, 2015;114:1294-303
- Pfrimer K, Vilela M, Resende CM, Scagliusi FB, Marchini JS, Lima NK, Moriguti JC, Ferriolli E, Under-reporting of food intake and body fatness in independent older people: a doubly labelled water study. Age Ageing. 2014;44:103-8
- Rennie KL, Coward A, Jebb SA, Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr, 2007;97:1169-76
- Fontana JM, Pan Z, Sazonov ES, McCrory MA, Thomas JG, McGrane KS, Marden T, Higgins JA, Reproducibility of Dietary Intake Measurement From Diet Diaries, Photographic Food Records, and a Novel Sensor Method. Frontiers Nutr. 2020;7:99
- Stice E, Palmrose CA, Burger KS, Elevated BMI and Male Sex Are Associated with Greater Underreporting of Caloric Intake as Assessed by Doubly Labeled Water. J Nutr, 2015;145:2412-8
- Trijsburg L, Geelen A, Hollman PC, Hulshof PJ, Feskens EJ, Van't Veer P, Boshuizen HC, de Vries JH, BMI was found to be a consistent determinant related to misreporting of energy, protein and potassium intake using self-report and duplicate portion methods. Public health Nutr. 2016;20:598-607
- Cena H, Calder PC, Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients. 2020;12(2):334. doi: 10.3390/nu12020334
- Schofield WN, Predicting basal metabolic rate, new standards and review of previous work. Human nutrition. Clin Nutr. 1985;39(S1):5-41
- Gibson RS, Charrondiere UR, Bell W, Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention. Adv Nutr. 2017;8:980-991
- Mendez MA, Popkin BM, Buckland G, Schroder H, Amiano P, Barricarte A, Huerta JM, Quirós JR, Sánchez MJ, González CA, et al. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol, 2011;173:448-58
- Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC, Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181:225-33
- Tooze JA, Freedman LS, Carroll RJ, Midthune D, Kipnis V, The impact of stratification by implausible energy reporting status on estimates of diet-health relationships. Biomet J. 2015;58:1538-1551
- Vainik U, Konstabel K, Lätt E, Mäestu J, Purge P, Jürimäe J, Diet misreporting can be corrected: confirmation of the association between energy intake and fat-free mass in adolescents. Br J Nutr, 2016;116:1425-1436
- Ejima K, Brown AW, Schoeller DA, Heymsfield SB, Nelson EJ, Allison DB, Does exclusion of extreme reporters of energy intake (the "Goldberg cutoffs") reliably reduce or eliminate bias in nutrition studies? Analysis with illustrative associations of energy intake with health outcomes. Am J Clin Nutr. 2019;110:1231-1239
- Black AE, Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. International journal of obesity and related metabolic disorders : J Int Assoc Study Obes, 2000;24:1119-30
- Banna JC, Fialkowski MK, Townsend MS, Misreporting of dietary intake affects estimated nutrient intakes in low-income Spanish-speaking women. J Academ Nutr Dietetics. 2013;115:1124-33
